What is an Intumescent Flame Retardant (IFR) System?
An Intumescent Flame Retardant (IFR) system is a condensed-phase mechanism, meaning it works within the solid polymer material itself, rather than in the gas phase (the flame).
The word “intumescent” means “to swell up.” When exposed to heat, an IFR system triggers a chemical reaction that causes the material to swell dramatically, forming a thick, porous, and stable layer of char (carbon).
This swollen char layer acts as a powerful insulating barrier, protecting the underlying polymer and stopping the fire.
The Three-Component “Recipe”
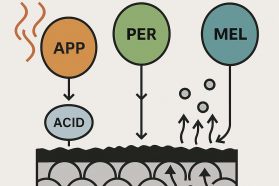
An IFR system is not a single chemical but a “recipe” that requires three distinct components to work together. If you are missing any one of these, the system will fail.
- The Acid Source (The “Catalyst”):
- What it is: A chemical that decomposes under heat to produce a strong, non-volatile mineral acid (like polyphosphoric acid).
- Common Example: Ammonium Polyphosphate (APP).
- The Carbon Source (The “Char-Former”):
- What it is: A polyol (a molecule with many -OH groups) that provides the carbon atoms needed to build the char layer.
- Common Example: Pentaerythritol (PER).
- The Gas Source (The “Blowing Agent”):
- What it is: A chemical that decomposes at the same time, releasing large volumes of non-flammable gases (like nitrogen and ammonia).
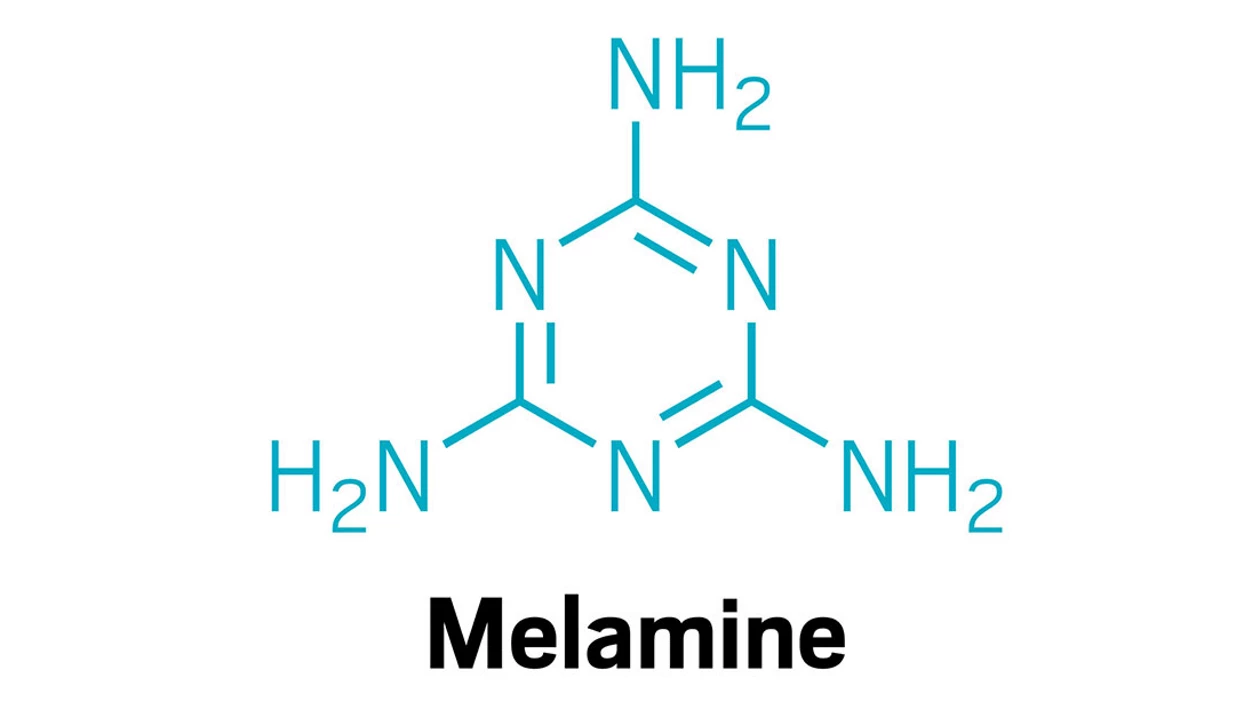
- Common Example: Melamine or Melamine Cyanurate (MCA).
The Mechanism: How Swelling Stops Fire
Imagine the IFR system as a “fire-activated foam factory” inside the plastic. When a flame hits the material, a precise chain of events occurs:
- Step 1: The Acid Source Activates
- At 250℃, the Acid Source (APP) decomposes, releasing polyphosphoric acid.
- Step 2: The Carbon Source is “Cooked”
- The hot polyphosphoric acid (a powerful dehydrating agent) attacks the Carbon Source (PER).
- It rips the hydrogen and oxygen atoms off the PER molecule, leaving behind a thick, sticky layer of black carbon “tar.” This is the beginning of the char.
- Step 3: The Gas Source Inflates the Char
- At a slightly higher temperature, the Gas Source (Melamine) decomposes, releasing a high volume of non-flammable gases.
- These gases bubble through the sticky carbon “tar” from Step 2, causing it to foam and swell—like bread rising in an oven.
- Step 4: The Barrier is Formed
- This expanded foam quickly solidifies into a thick, stable, and highly insulating layer. This intumescent char (which can be 50-100 times the original thickness) stops the fire in three ways:
- Heat Shield: It insulates the fresh polymer underneath, preventing it from reaching its ignition temperature.
- Fuel Block: It creates a physical barrier, trapping flammable gases from the decomposing polymer so they cannot escape to feed the flame.
- Oxygen Block: It prevents oxygen from the air from reaching the material.
- This expanded foam quickly solidifies into a thick, stable, and highly insulating layer. This intumescent char (which can be 50-100 times the original thickness) stops the fire in three ways:
Key Advantages
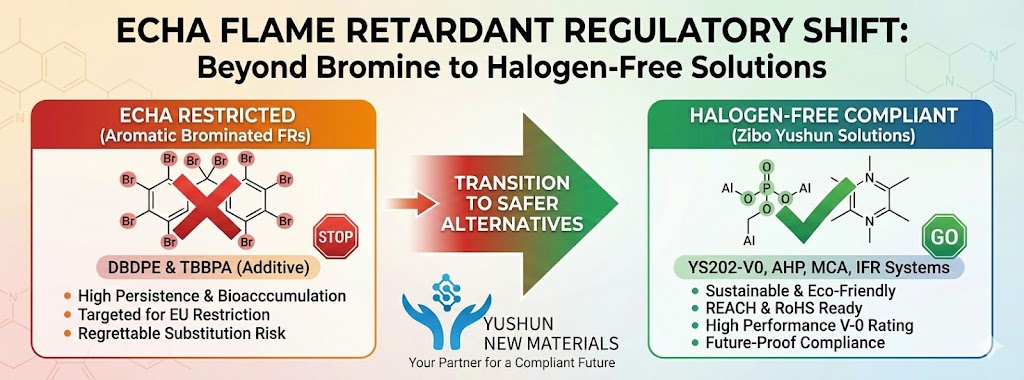
- Halogen-Free: IFR systems are a cornerstone of modern halogen-free flame retardancy.
- Effective in “No-Char” Polymers: Polymers like Polypropylene (PP), which (as we discussed) normally decompose completely and leave no char, can be forced to create a robust char layer using an IFR system.
- Low Smoke: Unlike many flame retardants (especially ATO), IFR systems are low-smoke and produce less toxic gas, as they trap the flammable components in the solid char.